World Health Organization PrequalifiedLevoplant,
Two-Rod Contraceptive Implant
Beijing, China, 12 July 2017 -- Shanghai Dahua
Pharmaceutical Co. Ltd. (Dahua), a
subsidiary of Bright Foods Group Changjiang General Corp. Ltd, is
pleased to announce that the World Health Organization (WHO) has prequalified Levoplant
also known as Sino-implant (II)– a highly effective and high quality two-rod
contraceptive implant – for three years of use. Dahua has manufactured two-rod
implants since 1996 and more than 11 million units have been distributed globally.
WHO Prequalification will now allow additional women worldwide to have access
to this high quality, highly effectivelong-acting reversible contraceptive (LARC) at affordable prices.
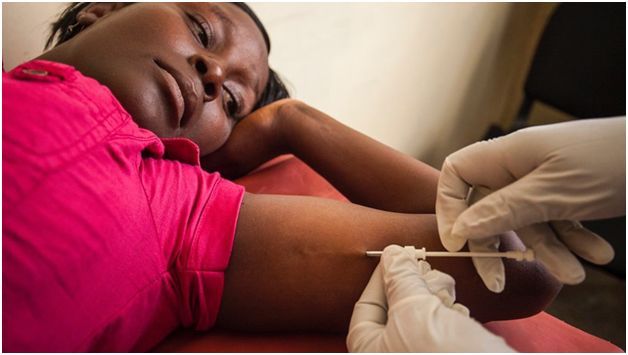
Photo shows
Millicent Vuguza receiving a Levoplant device from Maureen Olaka at the
Kariobangi North Health Center in Kariobangi, Nairobi, Kenya.
Voluntary
family planning is a vital component of global health. Access to contraceptives
results in fewer mothers dying in pregnancy and childbirth, fewer unintended
pregnancies, fewer abortions, and fewer infant deaths. But more than 220
million women in developing countries lack access to safe and effective
contraceptives. One of the barriers is a lack of sufficient supplies of appropriate,
affordable products that meet their needs.
“We are
delighted and honored to have achieved this important milestone.” said Mr. Bin Song, General Manager of Shanghai Dahua. “WHO Prequalification
will allow us to further increase our reach and impact globally. As a company,
we are committed to contribute to theFP2020 goal that targets to reach an
additional 120 million women
in low-resource settings with highly effective, high-quality and affordable
contraceptive services by 2020.Shanghai
Dahua is now the third WHO-prequalified LARC manufacturer, next to Merck (USA)
and Bayer (Germany).
Since 2007, the Bill & Melinda Gates Foundationhas
provided support to Dahua’s pursuit of WHO Prequalification through a project
led by FHI 360. In November 2016, following the WHO Expert Review
Panel process, the United Nations Population Fund (UNFPA) signed a one-year Long-term Agreement (LTA) to
procure with Dahua for purchasing Levoplant/Sino-implant
(II) by its country programs.Now, WHO
Prequalification of the product for three years of use will mean that
additional donors can purchase the product for country programs.
To date, Sino-implant(II)has been sold under brand name Levoplant.More informationfor
countries who are interested in introducing Levoplant can be found on Dahua’s website and in the K4Health Implants Toolkit.
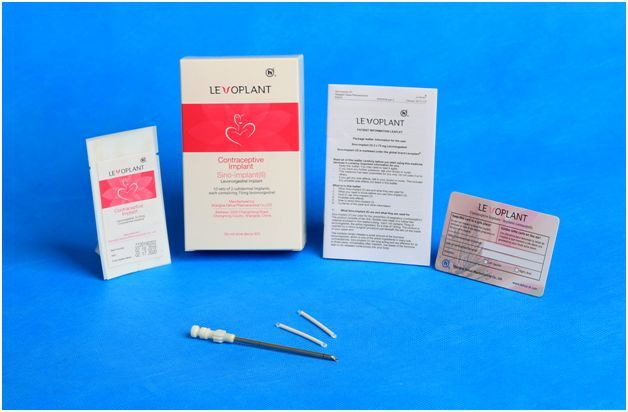
Levoplant


